De Priester Charts Calculator Online
K values from the De Priester charts, section 8.16.6. Determine Nm from equation 11.58. Determine Rm using equations 11.60, 11.61. Find N for a range of reflux ratios. Erbar and Madox method, Fig 11.1; see example 11.7. Select the optimum reflux ratio. Find the number of theoretical plates need at the optimum reflux. Math explained in easy language, plus puzzles, games, quizzes, worksheets and a forum. For K-12 kids, teachers and parents. Enable the calculations to be done using hand calculators, or ggp yraphically. Typical examples of this approach are the methods given. Equilibrium constants were taken from the De Priester charts. Relative volatilities estimated: Li ht k Light non-key comp.
DePriester Charts provide an efficient method to find the vapor-liquid equilibrium ratios for different substances at different conditions of pressure and temperature. The original chart was put forth by C.L. DePriester in an article in Chemical Engineering Progress in 1953. These nomograms have two vertical coordinates, one for pressure, and another for temperature. 'K' values, representing the tendency of a given chemical species to partition itself preferentially between liquid and vapor phases, are plotted in between.[1][2][3][4] Many DePriester charts have been printed for simple hydrocarbons.[5][6]
Example[edit]
De Priester Charts Calculator Online

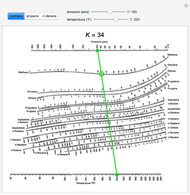
For example, to find the K value of methane at 100 psia and 60 °F.

Depriester Chart Calculator Online


- On the left-hand vertical axis, locate and mark the point containing the pressure 100 psia.
- On the right-hand vertical axis, locate and mark the point containing the temperature 60°F.
- Connect the points with a straight line.
- Note where the line crosses the methane axis. Read this K-value off the chart (approximately 21.3).
De Priester Charts Calculator online, free
References[edit]
De Priester Charts Calculator Online Calculator
- ^Dambal, Ajey. 'DePriester Charts'. Thermodynamics - Chemical Engineering (Washington University).
- ^'Pbub and Pdew calculation based on the DePriester chart for a fast pyrolysis and hydrotreating process'(PDF). Biofuelsacademy.org.
- ^McKetta Jr., John J. (24 September 1992). Unit Operations Handbook: Volume 1 (In Two Volumes). CRC Press, 1992. pp. 447–448. ISBN0824786696.
- ^'Define and use K values, relative volatility, and x-y diagrams'. The Pillars Curriculum for Chemical Engineering.
- ^McKetta Jr., John J. (24 September 1992). Unit Operations Handbook: Volume 1 (In Two Volumes). CRC Press, 1992. pp. 447–448. ISBN0824786696.
- ^Thammasat University. 'AE 335 Charts AE + SI'(PDF).